
Detoxification and Mode of Action of Plant Compounds in Herbivores
Plants use cocktails of defensive chemicals for protection against herbivore predators - indeed, many specialized metabolites are thought to have defensive roles as antifeedants or repellents. These include the relatively widespread phenolics, terpenes and alkaloids, as well as the more narrowly distributed glucosinolates, cyanogenic glucosides and benzoxazinoids, which are the main targets of our current work. Nevertheless, herbivores manage to use these well-defended plant tissues as food, and some crop pests even compete with humans for food sources. These successful herbivores are able to do so by employing, among other adaptations, detoxification strategies that lower or circumvent the toxicity of defensive phytochemicals. The chemistry and biochemistry behind these strategies are as varied and complex as the plant defensive cocktails, but are still quite poorly understood even in economically important crop-pest interactions. On the other hand, the molecular mechanisms through which plant chemical defenses exert their toxic effects are also in most cases still not identified. While some generalized modes of action have been proposed, the particular targets and the metabolic ramifications of intoxication have been largely ignored.
We are applying a variety of chemical and biochemical approaches to tackle these two aspects of plant-pest interactions. Our goals are to identify modes of action of plant defenses, as well as to elucidate and quantify corresponding detoxification pathways within the herbivores. Isotope labeling and tracing provide us crucial quantitative information on the importance of each detoxification pathway, and further enzymological and silencing/knock-down studies allow us to demonstrate the involvement of each pathway, and how they may benefit the herbivore. Proteomic analyses and assays in cultured cells help further clarify the processes directly and indirectly affected by plant-derived toxins.
Our current projects can be divided, based in the host-plant chemistry, in:
Glucosinolates
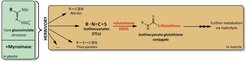
Cabbage, broccoli, radish, wasabi, and even the model plant Arabidopsis thaliana… all of these plants and others in the order Brassicales produce glucosinolates, the nitrogen- and sulfur-containing components of the so-called “mustard oil bomb”, an uncommon defense tactic against herbivores. Upon chewing-induced “detonation” of this bomb, a cascade of enzymatic chemical reactions takes place. Together, these transform otherwise harmless plant chemicals (glucosinolates) into potent feeding deterrents and even poisons, namely isothiocyanates, nitriles, and thiocyanates, among others. When humans bite into these plants, they experience a pleasant sharp flavor originating from glucosinolate hydrolysis products, whereas for smaller herbivores this bite can be toxic or deadly. Some herbivores, however, have biochemical means that enable them to exploit these plants as food sources. Elucidating the adaptive biochemical strategies of herbivores with respect to glucosinolates and the mechanisms of toxicity of these compounds are the main purposes of this project.
Role of glucosinolates in the diamondback moth-parasitoid interactions
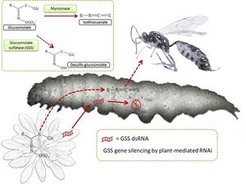
Certain hymenopteran parasitoids have been observed to differentiate between diamondback moth larvae feeding on the hostplants with different glucosinolate compositions. However, which individual glucosinolates are responsible for this differential response of parasitoids is not known. Similarly, whether the glucosinolate desulfation is beneficial or harmful to the larvae when they are under parasitoid attack is unknown. Ruo Sun proposes to answer these questions by studying the interaction between parasitoids and glucosinolate desulphatase-silenced diamondback moth larvae.
Glucosinolate detoxification strategies in phloem feeders in a multitrophic context
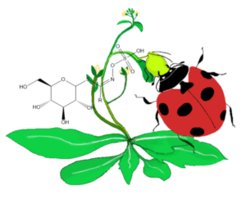
Plants from the Brassicaceae family defend themselves against herbivores by producing glucosinolate and its toxic breakdown products. Some herbivores, such as the phloem-feeding cabbage aphid Brevicoryne brassicae, have in turn evolved mechanisms to cope with these plant toxins, including even sequestration for their own defense against predators. Such herbivorous self-defense mechanisms constitute a possible route through which non-target organisms at higher trophic levels are exposed to plant defense compounds. The chemical ecology of such multitrophic interactions mediated by phloem-feeding herbivores is still scarce. For example, how predators of such a herbivore, such as ladybirds, metabolize the toxins present in their prey is largely unknown. Different ladybird species from the Coccinellidae family that appear to be more or less well-adapted to the toxins of Brevicoryne brassicae will allow us to better understand the major detoxification routes of glucosinolates in a multitrophic context. This project is being carried out as a part of the DFG-funded ChemBioSys Collaborative Research Center (chembiosys.de).
Cyanogenic glucosides

Cyanogenic glucosides (CNGlcs) are present as an activated chemical defense in many agriculturally relevant plants, such as the grass crops oats and maize, fruit crops such as apples and mangoes, and in the South American and sub-Saharan staple food cassava. Removal of a CNGlc sugar group leads to release of HCN, which inhibits cellular respiration leading to toxicity and death. We want to understand how detoxification of CNGlcs and other cassava defensive chemicals allows some sub-Saharan Bemisia tabaci whitefly strains to successfully and super-abundantly colonize cassava. Colonization by these phloem feeders leads to large losses in cassava production, either directly or via B. tabaci-vectored viruses.
This project is being performed in collaboration with Prof. Shai Morin and Dr. Osnat Malka from the Hebrew University of Jerusalem as part of a Bill & Melinda Gates Foundation-funded initiative led by the Natural Resources Institute of the University of Greenwich.
DIMBOA detoxification in fall armyworm (Spodoptera frugiperda)

The fall armyworm (FAW) is a notorious agricultural pest causing major damage to an array of agricultural cereal crops, corn being one of its important host plants. Corn produces a highly toxic defensive compound, the benzoxazinoid DIMBOA, that inhibits the growth of various insect pests feeding on the plant, but seems to be largely inefficient against FAW. In response to mechanical damage from herbivory, the plant releases DIMBOA from its storage (2R)-glucosidic form, but the FAW larvae effectively re-glucosylate the compound with a stereochemical shift that makes (2S)-DIMBOA-glucoside, an unsuitable substrate for defensive plant glucosidases still active in insect gut. This detoxification reaction is achieved in the insect by UDP-glucosyltransferases (UGTs), a highly versatile class of enzymes active against a broad range of compounds. We are currently trying to understand how critical this DIMBOA detoxification pathway in FAW is. The basic rationale involves silencing of UGT candidates that are expressed typically in response to DIMBOA and are highly substrate specific. The idea is to understand the interplay between host plant choice and the UGTs that may be playing the most important roles in conferring tolerance to DIMBOA, and how these enzymes are evolving in differential host usage context.




